Questions 28-34 are based on the following passage.
In a chemistry class, the teacher plac.ed 0.5 g of porous steel wool, composed mostly of iron (Fe), inside a small heat-resistant quartz tube. She then used silicone hoses to connect the quartz tube to 2 airtight glass syringes (see figure). Each syringe contained 8 mL of air, and the total volume of air in the closed apparatus was 20 mL.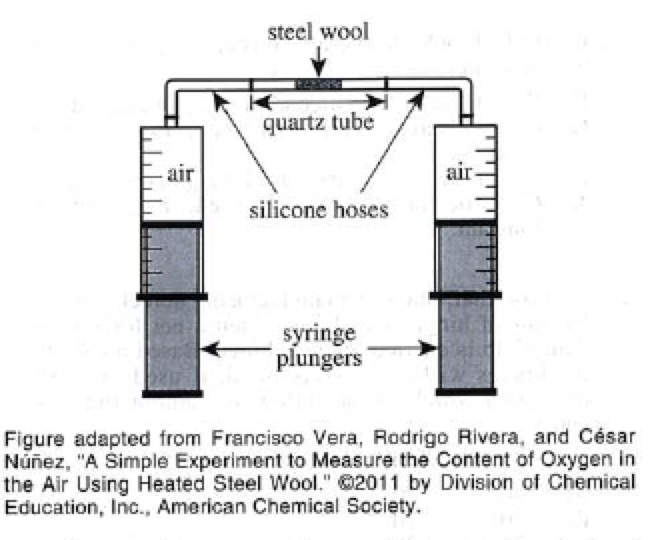
A Bunsen burner was then used to heat the contents of the quartz tube for 2 mm. Dunngneatmg, flie plungers were moved up and down to pass the air back and forth through the steel wool. The total volume of gas in the apparatus steadily declined over the 2 min. Once the apparatus and its contents returned to room temperature, the total volume of gas in the apparatus was 16 mL.
The teacher asked each of 4 students to explain What occurred during the demonstration.
Student 1
During heating, the Fe in the steel wol reacted with all ,the N₂ in the air to form solid iron nitride (FeN), which was deposited on the steel wool. Air contains about 20%
N₂ by volume. As a res11lt of the reaction, the total volume of gas in the apparatus decreased by about 20%, so almost all the gas remaining in the apparatus was O₂.
Student 2
During heating, the Fe in tlie steel wool reacted with some of the O₂ in the air to form solid iron oxide (Fe₂O₃), which was deposited on the steel wool. Air contains about 80% O₂ by volume. As a result of the -reaction, the total volume of gas in the apparatus decreased by about 20%. Almost all the gas remaining in the apparatus was a mixture of about 75% O₂ and 25% N₂ by volume.
Student 3
Student 2 is.correct, except that (1) the Fe in the steel wool reacted with all the O₂ in the air and (2) air contains about 20% O₂ by volume. After the reaction, almost all the gas remaining in the apparatus was N₂
Student 4
During heating, the Fe in the steel wool reacted with all the CO₂ in the air to form solid iron carbonate (FeCO₃), which was deposited on the steel wool. Air contains about 20% CO₂ by volume. As a result of the reaction, the total volume of gas in the apparatus decreased by about 20%, so almost all the gas remaining in the apparatus was O₂.