Questions 7-13 are based on the following passage.
A high concentration of dissolved nickel (Nᵢ²⁺) in wastewater is an environmental concern. Students studied the removal of Nᵢ²⁺ from wastewater, using ,an aqueous Nᵢ²⁺ solution as a model of wastewater.
In water, hydroxide (OH-) reacts with Nᵢ²⁺ to form nickel hydroxide monohydrate [Ni(OH)₂·H₂0]. The balanced chemtcal equation for this reactiol), is
Nᵢ²⁺ + 2OH⁻ + H₂O⟶Ni(OH)₂⋅H₂O
Because the monohydrate is a solid, it can be filtered from the solution. Some of the solid will eventually dissolve if it is left in contact with the solution.
The students did 2 experiments to study how reaction time and filtration method affected the removal of Nᵢ²⁺ from the aqueous Nᵢ²⁺ solution.
Experiment 1
In each of Trials 1—3, Steps 1—4 were performed:
1. Thirty-two mL of aqueous 1.0 mole/L OH⁻ solution and 260 mL of aqueous 0.060 mole/L Nᵢ²⁺ solution were poured into the same flask.
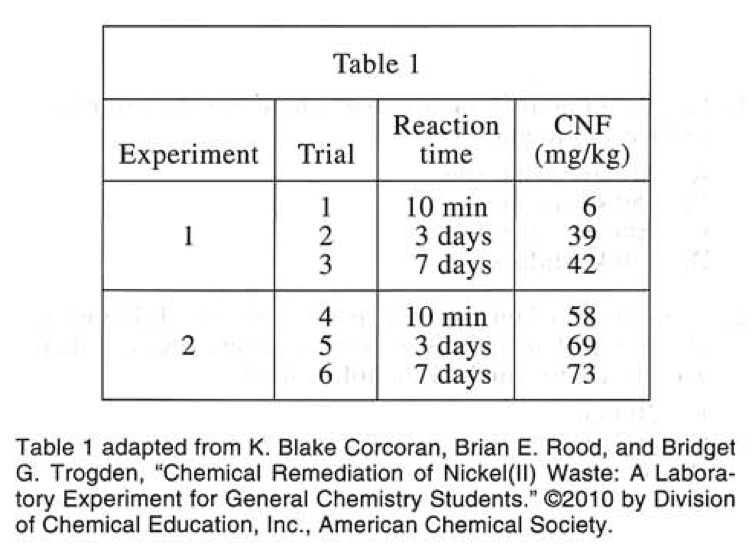
2. The mixture was stirred at 22°C. for 10 min; 3 days, or 7 days.
3. Solid monohydrate was recovered by standard filtration (see Figure 1) 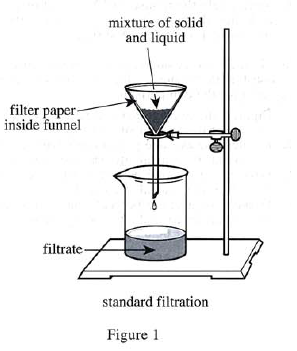
4. The concentration of Nᵢ²⁺ in the filtrate, CNF, was determined, in milligrams of Nᵢ²⁺ per kilogram of solution (mg/kg).
Experiment 2
In each of Trials 4—6, Steps 1—4 in Experiment 1 were performed except that in Step 3, solid monohydrate was recovered by vacuum filtration (see Figure 2). 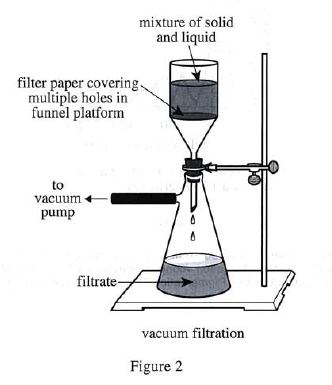
The results of Experiments 1 and 2 are shown in Table 1.