Questions 20-26 are based on the following passage.
The octane number of a fuel is a measure of how smoothly the fuel burns in a gasoline engine. Lower octane fuels knock (explode) when burned, which lowers fuel efficiency and can cause engine damage. Heptane. knocks considerably when burned and is given an octane number of 0. Isooctane knocks very little and is given an octane number of 100.
Different proportions of heptane and isooctane were mixed to obtain mixtures with octane numbers between 0 and 100 (see Table 1).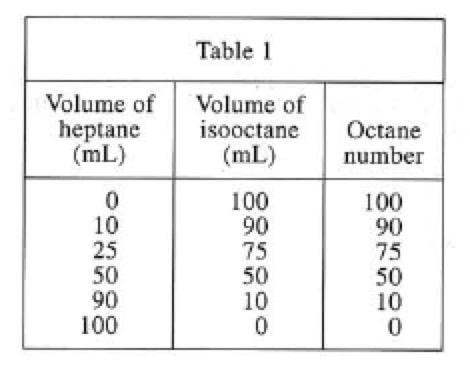
Experiment 1
A sample of each fuel mixture listed in Table 1 was burned in a test engine at an engine speed of 600 revolutions per minute (rpm). The number of knocks per minute was determined for each mixture. This was done so that an octane number could be assigned to any fuel by measuring its knock rate.
Experiment 2
Adding tetraethyllead (TEL) to a fuel changes its octane number. Different amounts of TEL were added to 1,000 mL samples of isooctane. Each fuel mixture was tested under the same conditions used in Experiment 1, and the measured knock rate was used to determine the octane number (see Figure 1).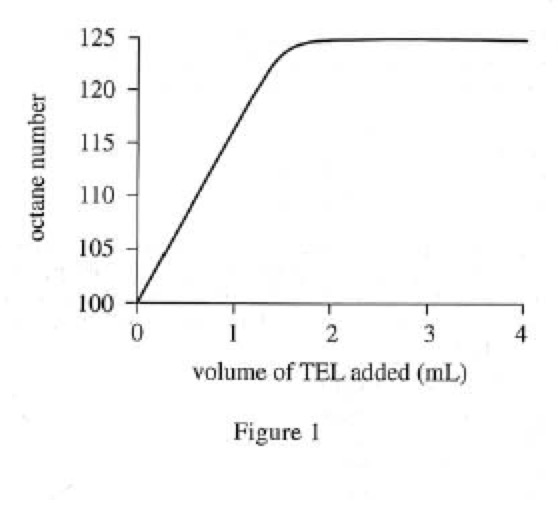
Experiment 3
The engine octane requirement (EOR) is the minimum octane number of a fuel required for an engine to operate without becoming damaged. Fuels A and B were burned separately in an engine at different speeds. Table2 shows the octane number determined for each fuel at each engine speed and the known EOR of the engine at each speed.