Questions 22-31 are based on the following
passage.
This passage is adapted from Rachel Ehrenberg, “Salt Stretches in Nanoworld.” ©2009 by Society for Science & the Public. The “nanoworld” is the world observed on a scale one billionth that of ordinary human experience.
Inflexible old salt becomes a softy in the
nanoworld, stretching like taffy to more than twice
its length, researchers report. The findings may lead
to new approaches for making nanowires that could
5 end up in solar cells or electronic circuits. The work
also suggests that these ultra-tiny salt wires may
already exist in sea spray and large underground salt
deposits.
“We think nanowires are special and go to great
10 lengths to make them,” says study coauthor
Nathan Moore of Sandia National Laboratories in
Albuquerque. “Maybe they are more common than
we think.”
Metals such as gold or lead, in which bonding
15 angles are loosey-goosey, can stretch out at
temperatures well below their melting points.
But scientists don’t expect this superplasticity in a
rigid, crystalline material like salt, Moore says.
This unusual behavior highlights that different
20 forces rule the nanoworld, says theoretical physicist
Krzysztof Kempa of Boston College. “Forget about
gravity. It plays no role,” he says. Surface tension and
electrostatic forces are much more important at this
scale.
25 Moore and his colleagues discovered salt’s
stretchiness accidently. They were investigating how
water sticks to a surface such as salt and created a
super-dry salt sample for testing. After cleaving a
chunk of salt about the size of a sugar cube with a
30 razor, the scientists guided a microscope that detects
forces toward the surface. When the tip was far away
there was no measured force, but within about seven
nanometers a very strong attraction rapidly
developed between the diamond tip of the
35 microscope and the salt. The salt actually stretched
out to glom on to the microscope tip. Using an
electron microscope to see what was happening, the
researchers observed the nanowires.
The initial attraction between the tip and salt
40 might be due to electrostatic forces, perhaps good old
van der Waals interactions,1 the researchers
speculate. Several mechanisms might lead to the
elasticity, including the excessive surface tension
found in the nanoworld (the same tension that allows
45 a water strider to skim the surface of a pond).
The surface tension is so strong that as the
microscope pulls away from the salt, the salt
stretches, Kempa says. “The inside has no choice but
to rearrange the atoms, rather than break,” he says.
50 This bizarre behavior is actually mirrored in the
macroworld, the researchers say. Huge underground
deposits of salt can bend like plastic, but water is
believed to play a role at these scales. Perhaps salty
nanowires are present in these deposits as well.
55 “Sodium chloride2 is everywhere—in the air, in
our bodies,” Moore says. “This may change our view
of things, of what’s happening at the nanoscale.”
The work also suggests new techniques for
making nanowires, which are often created through
60 nano-imprinting techniques, Kempa says. “We
invoke the intuition of the macroworld,” he says.
“Maybe instead of stamping [nanowires] we should
be nano-pulling them.”
Beginning of reading passage footnotes.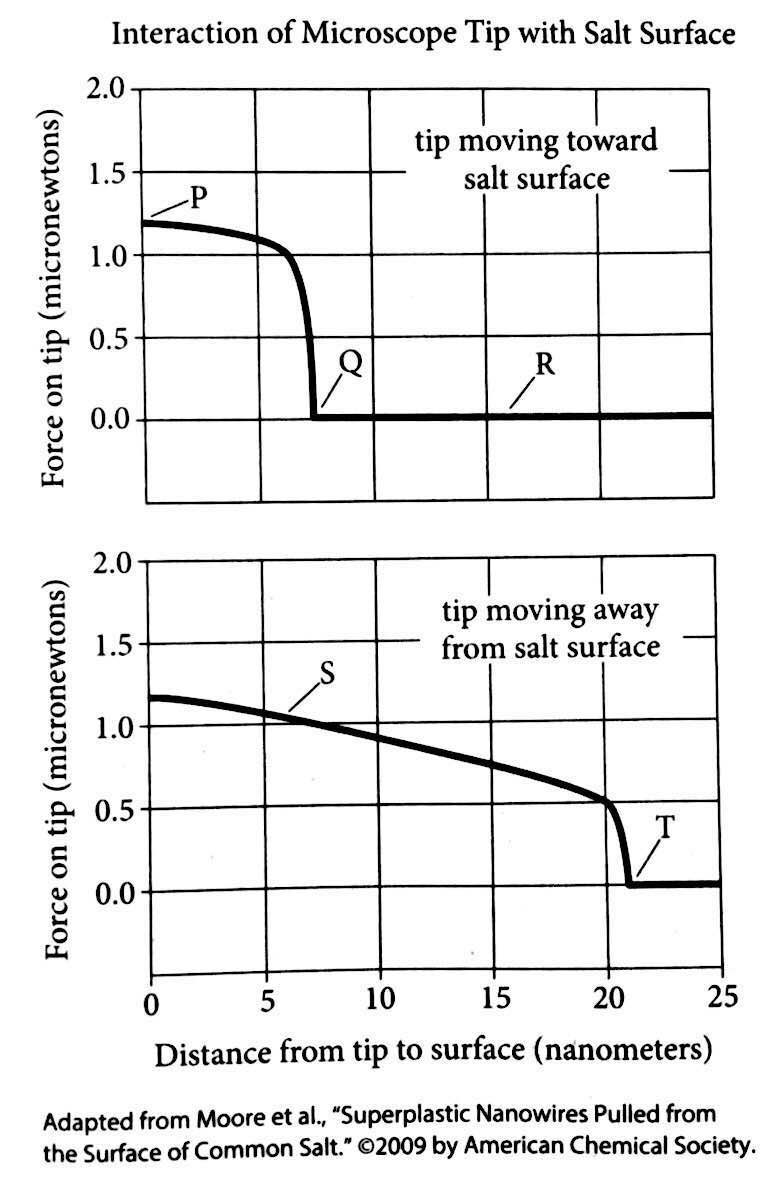
1 Attractive forces between nearby atoms
2 Common salt